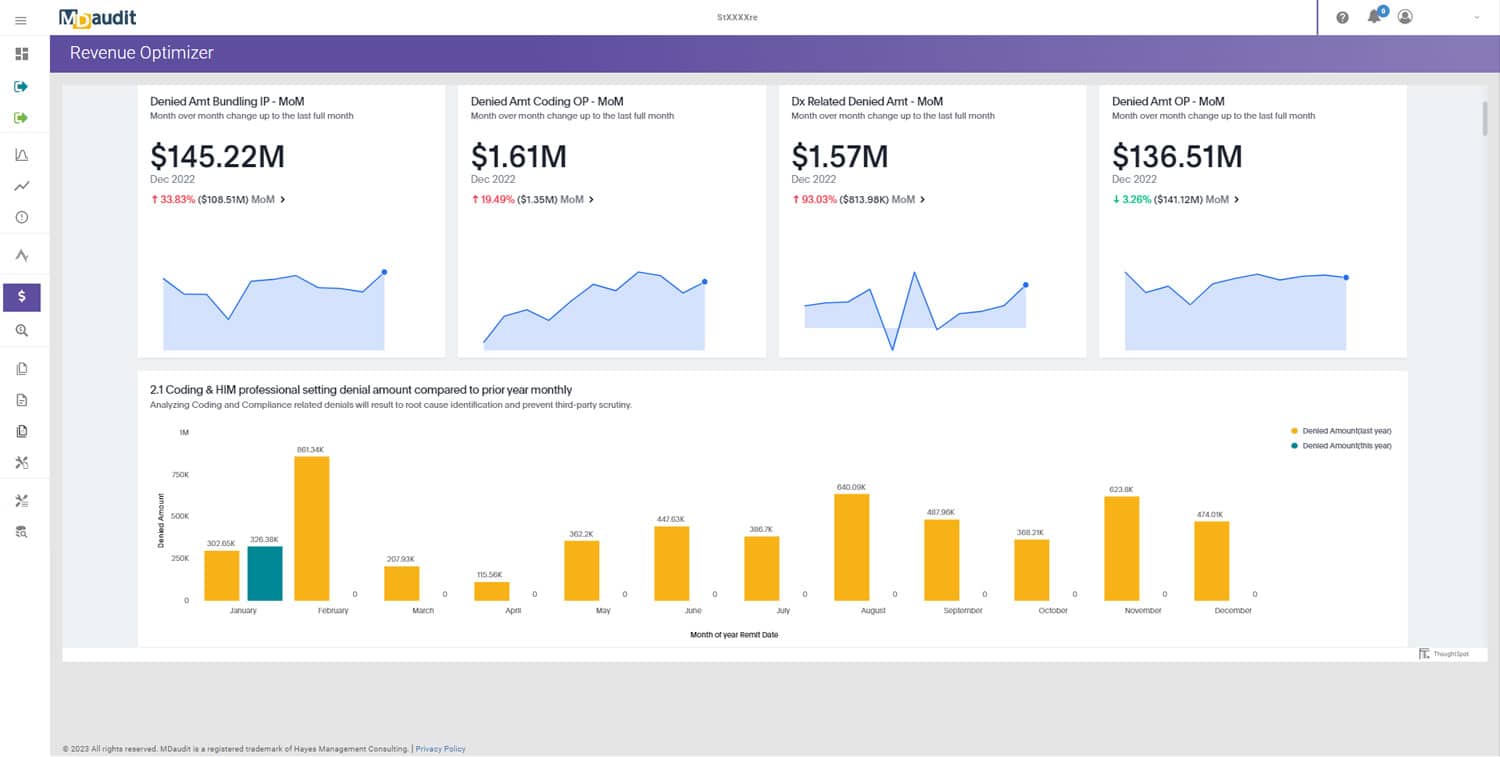
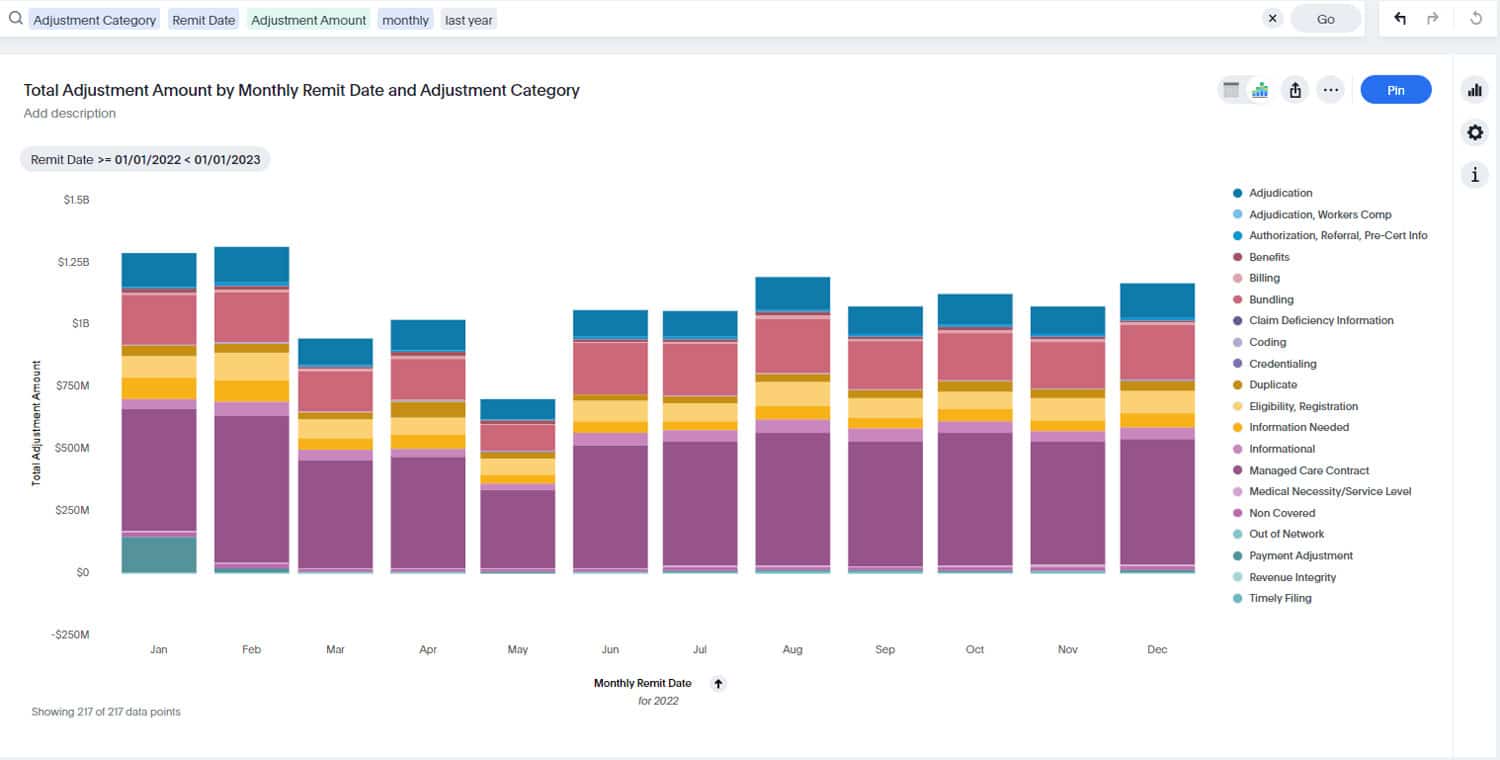
“The more things change, the more they remain the same.”
This French proverb is more relevant than ever when it comes to the proposed CMS changes for E&M documentation. Despite the exuberant celebration that greeted the announcement from CMS earlier this year that promised to reduce “documentation overload” for physicians, the reality is that the E&M documentation rules that have been around for over 20 years aren’t changing anytime soon.
The CMS documentation simplification will drive clinical operational changes for 2019, but it’s important to note that the 1995/1997 rules remain in full use now – and will likely stay in place even after the new proposal is implemented in 2021.
After collecting comments from the medical community over the past six months, CMS has decided to hold off on its proposed reforms for another two years. CMS Administrator Seema Verma says the delay offers more time for key stakeholders to provide input.
The main changes – cast as minor tweaks – affected the 99201 thru 99215 Office and Outpatient Visit codes and provided a new time-based reporting option for physicians. The proposal also simplified the history and examination elements of the three-point system for determining office visit codes, and allowed physicians to select their level of service for both new and established patients using only the medical decision-making (MDM) component. Finally, the original proposal altered the current five-level payment structure for new patients (99201-99205) and established patients (99211-99215) to just two levels.
So where do we currently stand? And what can we expect over the next few years and beyond? It’s pretty much ‘status quo’ for the near future, with the significant changes pushed back to 2020 and beyond.
Here’s what you should be prepared for year by year.
Changes in 2019 and 2020
Documentation requirements
For CY 2019 and CY 2020, CMS will continue to use the current coding and payment structure for E/M office/outpatient visits. Practitioners should continue to use either the 1995 or 1997 E/M guidelines to document E/M office/outpatient visits billed to Medicare.
In the meantime, CMS is working to finalize and clarify the following policies:
- Elimination of the requirement to document the medical necessity of a home visit in lieu of an office visit.
- For established patient office/outpatient visits, when relevant information is already contained in the medical record, practitioners may choose to focus their documentation on what has changed since the last visit, or on pertinent items that have not changed. This would eliminate the need to re-record the defined list of required elements if there is evidence that the practitioner has reviewed the previous information and updated it as needed. Practitioners should still review prior data, update as necessary, and indicate in the medical record that they have done so.
- For E/M office/outpatient visits, and for new and established patient visits, practitioners do not need to re-enter information in the medical record on the patient’s chief complaint or medical history if that information has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she has reviewed and verified this information.
- For E/M visits overseen by teaching physicians, elimination of the need to duplicate notations in medical records that may have previously been included by residents or other members of the medical team.
These proposals do not eliminate any documentation work on the two most important elements: Assessment and Planning. The changes would provide some documentation relief on patient history, but complete documentation of the examination along with the medical decision-making (MDM) remain key requirements. Practitioners should still review prior data and update as necessary. Documenting this action in the medical record is crucial.
These changes won’t result in much time savings. Obviously, using nurses and other staff to record as much of this information as possible could relieve physicians from some of the paperwork burden. But allowing other staff to help with documentation will require clinical process changes to minimize the risk of missing something important.
Practitioners are still required to use the 1995 or 1997 documentation rules with all key elements and requirements intact.
2021 and Beyond
Payment levels
The original proposal for E/M office/outpatient visits collapsed the existing five-level payment structure to just two levels. Level 1 would remain, and the remaining four levels would be paid out at approximately the midpoint between Levels 2 and 5. Following the comment period, CMS has changed this to a proposed three-level structure that basically leaves Levels 1 and 5 intact. Levels 2 through 4 will be paid out at a single rate for E/M office/outpatient visits for established and new patients.
Under the revised structure, the risk for audits now is more prominent than it was under the original version. Level 5 obviously will be scrutinized more heavily. As stated in previous posts on the proposed changes, even if the payment is the same for Levels 2 through 4, CMS will continue auditing for MDM and medical complexity. My guess is that the penalty for not meeting MDM requirements will be downgrading the claim to a Level 1 payment.
Time-based visit documentation
The proposal allows practitioners to use time or MDM to document E/M office/outpatient level 2 through 5 visits, instead of applying the current 1995 or 1997 E/M documentation guidelines. On the one hand, the rule states that practitioners can shift to time-based visit documentation, but the option to continue using 1995/1997 rules is still there. However, when time-based documentation is used, practitioners will still have to document the medical necessity of the visit and note that they personally spent the required amount of face-to-face time with the patient.
This provision of the new rule is still fairly vague, so how clinicians would handle this change is still a question. One thing is clear: this new rule will NOT be as simple at it seems. Practitioners who choose time-based documentation will still need to provide details on the MDM and the supporting time spent. This component will surely require further discussion and explanation by CMS.
Add-on codes
Feedback from clinicians noted that certain Level 2 to Level 4 visits might require additional codes to properly document services that go above and beyond the average E/M visit. CMS agreed and added a new provision to the proposal to allow the use of add-on codes that describe the additional resources needed for visits for primary care and certain non-procedural specialized medical care. These add-on codes, however, would be restricted by physician specialty.
These codes would only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally wouldn’t impose new per-visit documentation requirements. When physicians need to spend extended time with patients during E/M office/outpatient level 2 through 4 visits, they could also use a new “extended visit” add-on code to account for the additional required resources.
CMS added this component to the proposal to provide practitioners with greater flexibility to exercise clinical judgment in documentation, and enable them to focus on issues that are clinically relevant and medically necessary. CMS intends to solicit additional public input to possibly refine the policies further for CY 2021.
While at first blush this additional flexibility would seem beneficial, in my experience using add-on codes tends to raise red flags – and attract added scrutiny – with CMS, since the bottom line is that they have to pay more. There should be further clarification when specific guidelines come out, but the issue of using add-on codes does pose significant challenges. For instance, how will a clinician differentiate between an “add on” to a Level 4 versus a Level 5. As additional feedback comes in, you can be sure this provision of the proposal will look much different by the end of 2020.
Basics Still Apply
The CY’21 release coincides with the new quality-based guidelines, which is not an accident. The timing reinforces the fact that as much as CMS is attempting to simplify the requirements, the need for comprehensive and accurate data, correct coding, and robust documentation is not going away. If anything, auditing and analytics remain as intense as ever since the rules – including the new changes – remain complex.
The CMS documentation simplification will drive clinical operational changes for 2019, but it’s important to note that the 1995/1997 rules remain in full use now – and will likely stay in place even after the new proposal is implemented in 2021. This means audit scrutiny and compliance risks remain high – so it’s important to protect yourself against penalties and reimbursements by continuing to use your analytics tools and upgrading your systems to keep pace. This is your best approach, since the “nirvana” of documentation simplification appears to be far off in the future.